Self-powered sensors are key to more accurate, continuous health monitoring.
The researchers of Penn State like Huanyu “Larry” Cheng, Dorothy Quiggle Career Development Assistant Professor of Engineering Science and Mechanics are working to improve health monitoring by creating wearable sensors that collect data for clinicians while limiting discomfort for patients.
They are working on novel components and approaches to develop such devices as:
- wearable head scanners
- needle-free glucose monitors
- wearable antennas
- printable electronics.
The sensors are
- made with flexible electronics
- capable of monitoring patients’ physical motions and chemical signals in their sweat, skin
- more to help diagnose or inform treatment plans
- and the key is to make devices sustainable, resilient and self-charging.
Self-powered, rechargeable wearables
Developing flexible, economical sensors is one thing but powering them is another.
Although self-charging power units for stretchable energy harvesters already exist, they are expensive to fabricate, heavy to carry and they suffer from low and unstable output power.
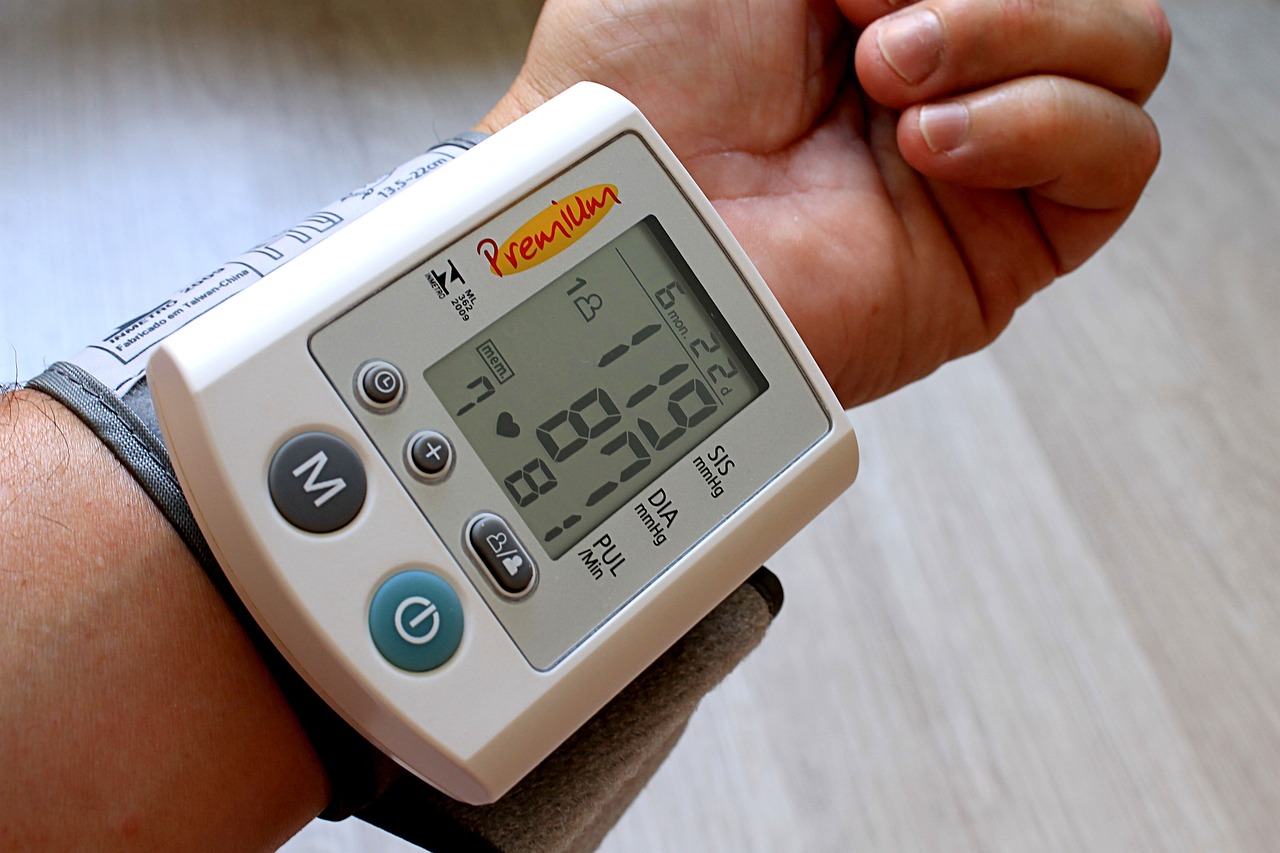
Here graphene material could help. It can harvest energy from motion, such as human body movements, and store it as electrical energy in micro-supercapacitors and as other types of wearable sensors, the self-powered device can measure users’ vital signs such as pulse, temperature, electrocardiogram, blood pressure and blood oxygen.
Tissue paper for pressure readings
Tissue paper decorates presents, protects breakables and, thanks to the research team, it can monitor blood pressure and respiratory conditions. A wearable sensor that detects blood pressure and movement can use a small skin patch built from inexpensive, widely available tissue paper. The wireless device adheres comfortably to a user’s forearm and reads blood pressure by measuring the dilation and constriction of a blood vessel in the wrist.
A sensor for humid environments
Most wearable sensors use superhydrophobic materials to repel water, but they have limited flexibility and often degrade quickly in humid environments.
To solve that problem you could combine superhydrophobic materials with Joule heating, where electric current passes through a conductor to produce heat. The heat provides continuous moisture resistance, even when the sensor is in an environment with 99% humidity.
The result is a new flexible pressure sensor that is able to withstand high degrees of humidity. The details were made available online ahead of the March issue of Chemical Chemical Engineering Journal..
Outlook
“We don’t know where inspiration will strike — tissue paper, lotus plants, motion power and more have all proved fruitful sources,” Cheng said. “From discovery to research to application, our team is enjoying the challenge of creating the next generation of medical devices.”
Here the link to the full interesting article of Penn State – Pennsylvania State University https://www.psu.edu/news/engineering/story/new-research-advances-wearable-medical-sensors/