Cardeology, more commonly referred to as cardiology, is the medical specialty focused on the diagnosis, treatment, and management of diseases and conditions of the heart and blood vessels. It involves a broad range of diagnostic and therapeutic procedures supported by various medical devices specifically designed for cardiovascular care.
Cardiology and Cardiologic Medical Devices
Overview of Cardiology
Cardiology encompasses the study and treatment of heart diseases, including arrhythmias, heart failure, coronary artery disease, and valvular heart conditions.
The field uses both non-invasive and invasive methods to diagnose and treat patients, employing a variety of medical devices to monitor, support, or correct heart function.
Types of Cardiologic Medical Devices
Cardiologic medical devices can be broadly categorized into diagnostic, monitoring, interventional, and implantable therapeutic devices:
Diagnostic and Monitoring Devices:
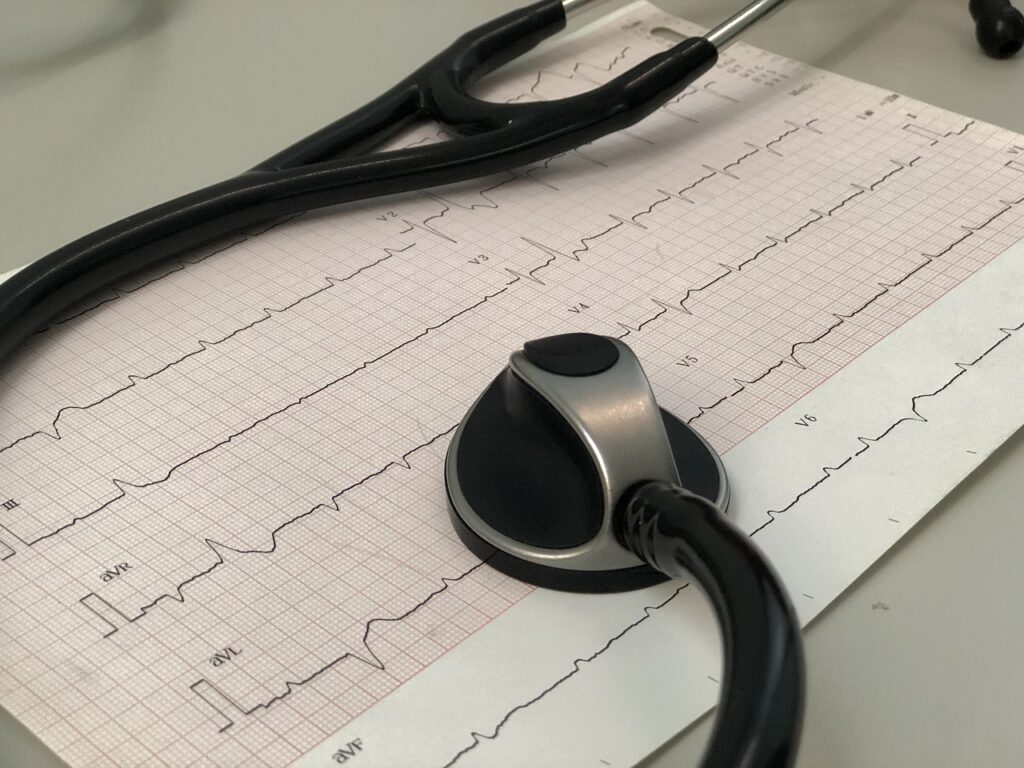
- Stethoscopes and sphygmomanometers for basic clinical assessment.
- Electrocardiograph (ECG) machines to record electrical activity of the heart.
- Cardiac imaging systems such as echocardiography and cardiac MRI.
- Implantable Loop Recorders (ILR) that continuously monitor heart rhythms over long periods.
Implantable Therapeutic Devices:
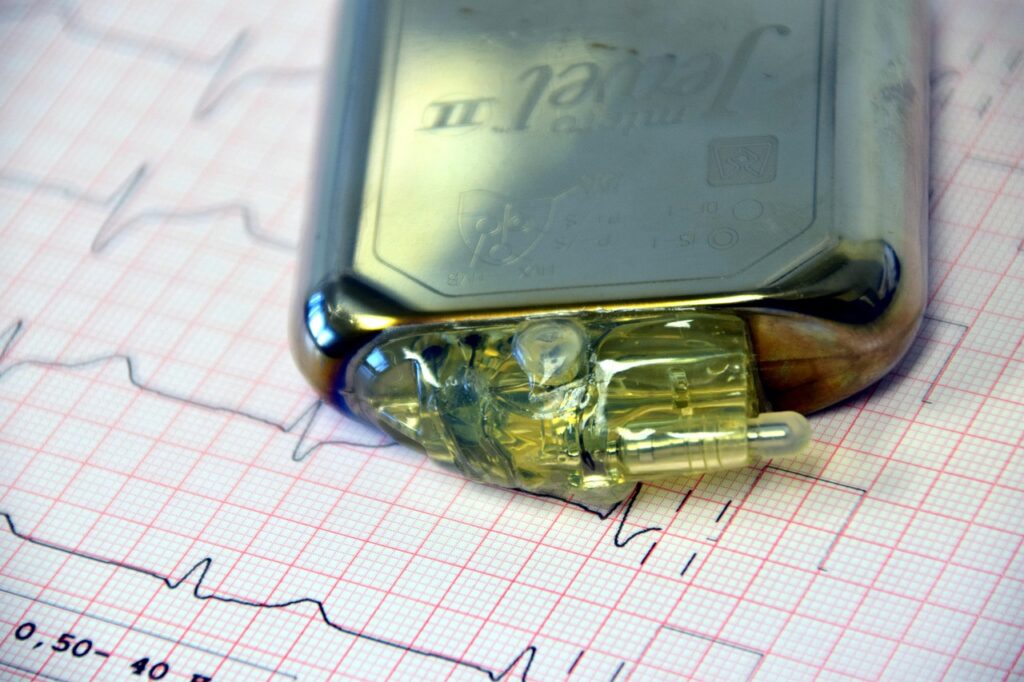
- Pacemakers that regulate slow heart rhythms by delivering electrical pulses.
- Implantable Cardioverter-Defibrillators (ICDs) that detect and correct dangerous arrhythmias by delivering shocks.
- Cardiac Resynchronization Therapy (CRT) devices that coordinate contractions in heart failure patients to improve cardiac output.

Interventional Devices:
- Coronary and peripheral stents to keep blood vessels open.Balloon catheters for angioplasty procedures.
- Ablation catheters used in electrophysiology to destroy abnormal heart tissue causing arrhythmias.
- Guidewires, vascular sheaths, and closure devices facilitating minimally invasive procedures.
Structural Heart Devices:
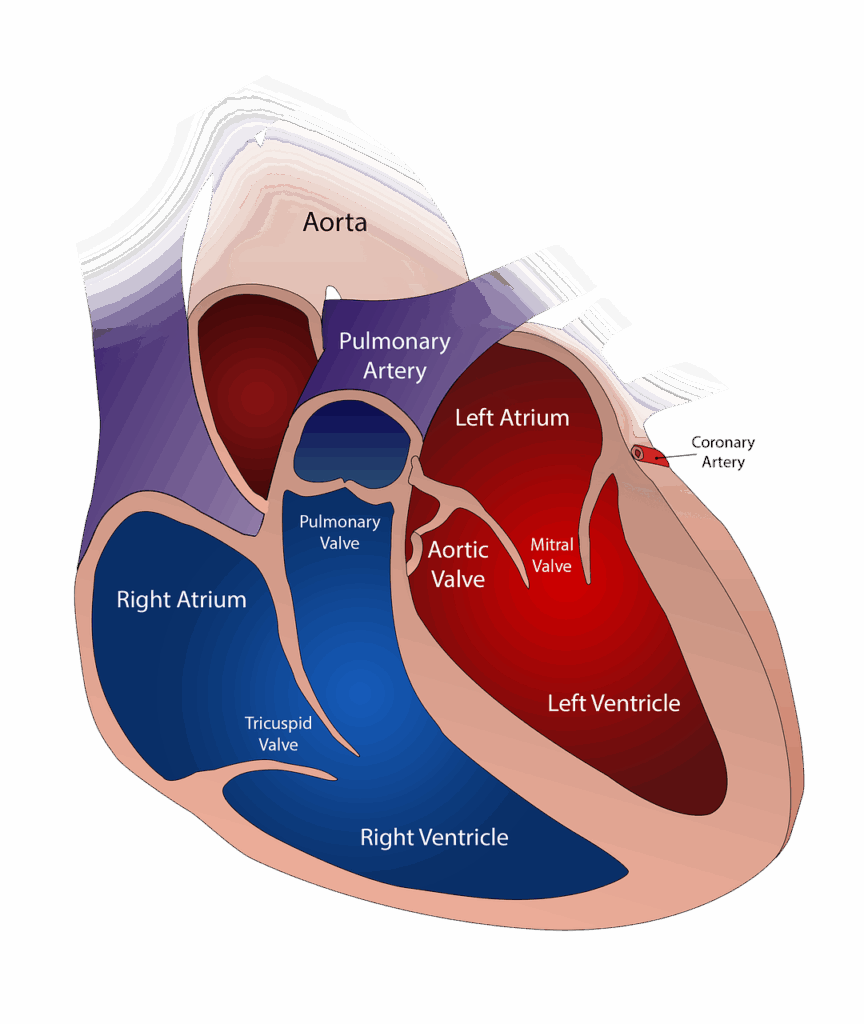
- Prosthetic heart valves and valve repair devices including transcatheter valve replacement systems AVR, TMVR).
- Artificial hearts and ventricular assist devices (VADs) that support or replace heart function in severe heart failure.
Regulatory and Safety Aspects
Cardiologic devices are often classified as high-risk medical devices, requiring stringent regulatory oversight to ensure safety and efficacy. In the European Union, the Medical Device Regulation (MDR) governs the approval and monitoring of these devices, with expert panels including cardiologists contributing to device assessments.
Similarly, the U.S. FDA conducts regulatory science research focused on cardiovascular devices, addressing challenges such as durability, hemocompatibility, and electrophysiology safety to improve preclinical testing and clinical outcomes.
Innovation and Market Trends
The cardiology medical devices market is highly competitive and innovative, with major companies like Medtronic, Abbott, and Boston Scientific leading development. Recent advances include AI-powered diagnostic tools such as Ultromics EchoGo Heart Failure, which uses deep learning to improve detection and management of heart failure with preserved ejection fraction (HFpEF), a condition traditionally difficult to diagnose.
Summary
Cardiology relies heavily on a diverse range of medical devices for diagnosis, treatment, and management of heart diseases. These devices include both non-invasive diagnostic tools and implantable devices like pacemakers and defibrillators, as well as interventional and structural heart devices. Regulatory frameworks ensure these devices meet safety and performance standards, while ongoing innovations continue to improve patient outcomes in cardiovascular care.
This summary draws on detailed information about cardiology instruments and devices, market segmentation, regulatory frameworks, and recent innovations in the field.
Sources
https://en.wikipedia.org/wiki/Instruments_used_in_cardiology
https://alirahealth.com/wp-content/uploads/Cardiology-Medical-Devices-Market-Trends-2023.pdf
https://nyulangone.org/conditions/cardiac-device-management/types
https://www.medicalexpo.com/cat/cardiology-GT.html
https://www.escardio.org/The-ESC/Advocacy/medical-device-regulation
https://www.barringtonjames.com/resources/blog/how-the-medical-device-industry-is-shaping-cardiology-innovation/
https://www.tuvsud.com/en/industries/medical-devices/cardiovascular-medical-devices












